Farm & Ranch
Theileria orientalis Genotype Ikeda: an Emerging Cattle Disease?

Theileria orientalis genotype Ikeda is a hemoprotozoan. A hemoprotozoan affects red blood cells and leukocytes. T. orientalis genotype Ikeda has been associated with severe bovine disease in Japan, New Zealand and Australia (Watts, Playford, & Hickey, 2016). In August of 2017, the protozoan was found associated with illness and death in cattle at a ranch in Virginia (Oakes et al., 2019). These cattle displayed clinical signs of weakness and anemia. A preliminary diagnosis of anaplasmosis was given. Blood samples taken from the animals were tested for Anaplasma, Babesia and Leptospira. Results from the test revealed the cattle were all negative; however, a blood protozoan was detected. This protozoan was identified as T. orientalis genotype Ikeda (Oakes et. al, 2019). Since this original herd outbreak, other herds of cattle in Virginia and West Virginia have been found to be infected with the organism.
In Australia, the Asian longhorn tick (Haemophysalis longicornis) has been identified as a possible vector of T. orientalis genotype Ikeda (Hammer, Emery, Bogema, & Jenkins, 2015). In 2017, the United States Department of Agriculture’s National Veterinary Services Laboratories confirmed the presence of Haemaphysalis longicornis, which is commonly referred to as the Asian longhorn tick or bush tick. In an effort to understand how the tick got to the United States, USDA officials discovered the tick had been found in West Virginia in 2010. The tick has been confirmed to be in Arkansas, Connecticut, Delaware, Kentucky, Maryland, New Jersey, New York, North Carolina, Pennsylvania, Tennessee, Virginia, and West Virginia (USDA, 2020). Some evidence exists for other possible insect vectors for T. orientalis genotype Ikeda. Needle transfer is another possible route of transmission of the organism (Watts, Playford, & Hickey, 2016).
If the Asian longhorn tick is ever found in Oklahoma, Dr. Justin Talley, Oklahoma State University Extension livestock entomologist, believes the tick will live east of I-35. The tick will likely be found in vegetation that is high in humidity in areas such as woods, brush or tall grass. Ticks are usually found where large numbers of wildlife congregate such as a deer trail. Dr. Talley and Dr. Bruce Noden have written an excellent fact sheet about the longhorn tick. The fact sheet can be found at http://entoplp.okstate.edu/pddl/2018/PA%2017-16.pdf.
Cattle infected and sick with T. orientalis genotype Ikeda will have clinical signs of fever, weakness, anorexic, and exercise intolerance. If cattle are forced to move, they may stagger and gasp for air. If stressed too much, the cattle may collapse and die. When examining cattle, the gums, eyes or vaginal mucosa may appear white or yellow in color. Reproductive losses including stillbirths, and late term abortions may be seen as well as reduction in milk production (Spickler, 2019). Since Anaplasma marginale and T. orientalis genotype Ikeda both display similar clinical signs, one difference that has been noted in the two diseases is A. marginale infected cattle usually display aggression and T. orientalis genotype Ikeda do not. Still, a laboratory test would have to be performed to differentiate the two diseases.
In other countries, therapies have been developed to treat this organism. Unfortunately, no approved treatments are available in the United States. Also, there are no vaccines for this disease. The best defense to this disease is to control ticks. This usually requires a combination of insecticide treatments and pasture rotation to avoid areas such as woods and brushy areas where ticks live.
Theileria orientalis genotype Ikeda is not likely to arrive in Oklahoma anytime soon and in reality, may never become a major problem in Oklahoma or the United States. However, producers need to keep in mind the natural progression of cattle in the United States is east to west and south to north. Oklahoma is unique in the fact large numbers of cattle move from the southeast United States to graze on grass and wheat in our state. From there, the cattle go to the feedyards. The tick and organism could easily be transported to Oklahoma on a load of stockers headed for grazing or to a feedlot. If a producer would like more information on T. orientalis genotype Ikeda, they should contact their local veterinarian or Oklahoma State University Extension Educator or visit the Center for Disease Control and Prevention at https://wwwnc.cdc.gov/eid/article/25/9/19-0088_article.
References
Hammer, J. F., Emery, D., Bogema, D. R., & Jenkins, C. (2015). Detection of Theileria orientalis genotypes in Haemaphysalis longicornis Ticks from Southern Australia. Parasites & vectors, 8, 229.
Oakes, V. J., Yabsley, M. J., Schwartz, D., LeRoith, T., Bissett, C., Broaddus, C., Schlater, J. L., Todd, S. M., Boes, K. M., Brookhart, M., & Lahmers, K. K. (2019). Theileria orientalis Ikeda Genotype in Cattle, Virginia, USA. Emerging infectious diseases, 25(9), 1653–1659.
Spickler, Anna Rovid. (2019).Theileriosis. Retrieved from http://www.iastate.edu/DiseaseUbfi/factsheets.php.
Watts, J. G., Playford, M. C., Hickey, K.L. (2016). Theileria orientalis: A Review, New
Zealand Veterinary Journal, 64:1, 3-9.
Read more great stories in the April 2020 issue of Oklahoma Farm & Ranch.
Farm & Ranch
Hazards of Backyard Poultry

Barry Whitworth, DVM, MPH
Senior Extension Specialist, Department of Animal & Food Sciences, Ferguson College of Agriculture
Having backyard poultry is a popular agriculture enterprise. According to the United States Department of Agriculture, 0.8 percent of all households in the United States have chickens. People keep chickens for a variety of reasons with table eggs being one of the more common reasons. Unfortunately, some of these poultry producers are not aware of the hazards that come with keeping poultry because many times they carry pathogens but appear healthy.
Chickens are carriers of several zoonotic diseases. These are diseases that can be passed from animals to humans. According to a recent survey in Pennsylvania, a majority of backyard poultry producers were aware of the dangers of avian influenza. However, this study also revealed that far fewer producers were aware of the risk of possible exposure to Salmonella and Campylobacter. The lack of knowledge about the hazards of raising poultry likely contributes to the continued issues of Salmonella outbreaks associated with backyard poultry. In 2023, the Centers for Disease Control and Prevention reported 1,072 illnesses of Salmonella linked to backyard poultry, and 272 of those patients required hospitalization. Oklahoma reported 43 individuals with the disease.
Direct contact with chickens is not the only way to be exposed to the pathogens they carry. The environment in which they live can be a danger due to air quality and waste in the soil. The air in a poultry coop is composed of dust particles, ammonia, pathogens, poultry droppings, and other materials. Breathing the dust while cleaning a poultry coop has been associated with respiratory issues in poultry workers. One study found that human infections are associated with contact with poultry waste and soil. Backyard poultry producers may be exposed to poultry droppings when cleaning equipment or pens.
Most zoonotic diseases can be prevented. Proper hand hygiene is one of the best disease prevention tools available. According to the Pennsylvania study, most poultry producers wash their hands after having contact with their birds. However, that same study found most poultry producers do not wear gloves or cover their mouths when handling animals or animal manure. Backyard poultry producers should wear proper protective equipment when cleaning equipment and pens.
Poultry producers can protect themselves by following some simple rules.
- Wash hands with soap and water before and after having any contact with poultry or any area where poultry are located. If soap is not available, use hand sanitizer.
- Do not kiss or snuggle birds.
- Do not allow poultry to enter areas where food and drinks are prepared, served and stored.
- Do not eat or drink where poultry are located.
- Cook eggs thoroughly.
- Clean equipment associated with poultry outdoors.
- Older adults, pregnant women, children under five, and immunocompromised individuals should be extra careful around poultry.
- Wear protective clothing, shoes, gloves, and a face mask when cleaning poultry houses.
Having chickens in the backyard can be very rewarding experiences. However, poultry owners should be aware of the potential hazards associated with backyard poultry production and protect themselves. If poultry producers would like more information about hazards associated with backyard poultry, contact your local veterinarian and/or Oklahoma State University County Extension Agriculture Educator. Also, the CDC has a website dedicated to backyard poultry producers’ health. The website can be accessed at https://www.cdc.gov/healthypets/pets/farm-animals/backyard-poultry.html.
Farm & Ranch
Inventions of Agriculture: The Reaper
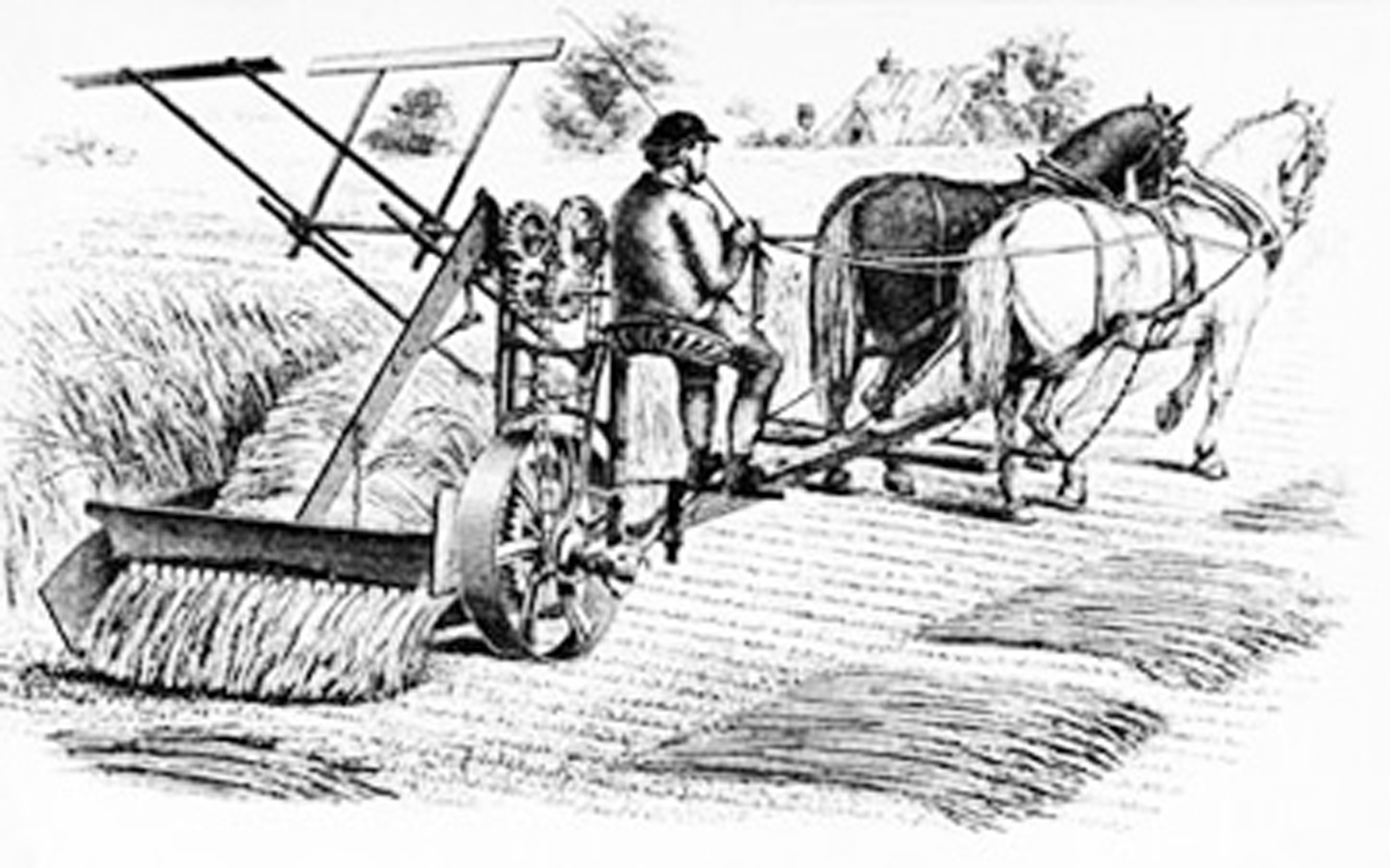
Agriculture has been a staple of human society since around 9000 BCE during the Neolithic Era, when humans began developing and cultivating their own food.
For centuries, food production was a slow, tedious process until the invention of agricultural machinery. One such invention was the reaper. Until its time, small grains were harvested by hand, cut with sickles or scythes, hand-raked and tied into sheaves.
While a few had unsuccessfully attempted to create a similar machine, it was Cyrus McCormick who would ultimately be credited with the invention of the first commercially successful reaper in 1831.
McCormick’s invention was a horse-drawn machine used to harvest wheat, a combination between a chariot and a wheelbarrow. He had joined together the earlier harvesting machines into a single, timesaving one. His reaper allowed producers to double their crop size, capable of cutting six acres of oats in just one afternoon. In contrast, it would have taken 12 workers with scythes to do the equivalent in the same amount of time.
McCormick had simply followed in his father’s footsteps. Growing up in Rockbridge County, Virginia, his father had also created several farming implements and even worked to invent a mechanical reaper of his own.
McCormick would patent his invention in July 1834, a year after Obed Hussey had announced the making of a reaper of his own. In 1837, McCormick began manufacturing his machine on his family’s estate.
In 1847, McCormick recognized Chicago as the future of the agricultural machinery industry. The railroad to Galena was nearing completion, the Illinois and Michigan Canal would soon be open, and a telegraph link to the east was coming. So, in 1847, McCormick, together with his partner and future Chicago mayor Charles M. Gray, purchased three lots on the Chicago River and built a factory where they would produce the reaper. It was the first of many industrial companies that would make their way to the area, making Chicago an industrial leader.
McCormick wasn’t done yet. He purchased an additional 130 acres in Chicago in 1871, but the Great Fire of 1871 threatened to destroy his company when the factory burned. It was his young wife, Nettie Fowler McCormick, who pushed the company forward when she went to the site just days after the fire and ordered the rebuilding of the factory. By 1880, McCormick was the largest machinery producer in Chicago and employment reached 7,000, a whopping fifth of the nation’s total.
McCormick joined the companies of Deering and Plano to form the International Harvester Company in 1902. At its height, the company controlled more than 80 percent of grain harvesting equipment in the world. While the Great Depression would hit Chicago’s agricultural industry hard, McCormick’s invention of the reaper forever changed the face of agriculture.
Resources
Carstensen, Fred. (2005) Agricultural Machinery Industry. Encyclopedia of Chicago. Retrieved from http://www.encyclopedia.chicagohistory.org/pages/29.html
Cycrus McCormick, Mechanical Reaper. (2022) The National Inventors Hall of Fame. Retrieved from https://www.invent.org/inductees/cyrus-mccormick
Although the author has made every effort to ensure the information in this article is accurate, this story is meant for informational purposes only and is not a substitute for historical documents.
Farm & Ranch
Scrapie

Barry Whitworth, DVM
Senior Extension Specialist Department of Animal & Food Science Ferguson College of Agriculture
Scrapie is a chronic, progressive disease of the central nervous system that affects sheep and goats. Scrapie is the oldest of the group of neurodegenerative diseases known as transmissible spongiform encephalopathies (TSE). Some of the other TSE are Bovine Spongiform Encephalopathy known as mad cow disease, Chronic Wasting Disease which is found in deer, and Creutzfeldt Jacob Disease which is found in humans. TSE are protein-misfolding diseases that lead to brain damage and are always fatal.
The cause of Scrapie is not completely understood, but evidence indicates that an infectious protein referred to as a prion is responsible for the disease. These infectious prions cause damage to the normal prion proteins found in the brain. The mis-folding of the proteins lead to brain damage and the presentation of clinical signs of the disease. Prions are very resistant to destruction, so once in the environment, they are difficult to remove.
Scrapie is believed to primarily be transmitted by the oral route. Typically, lambs and kids might ingest the prion when they come in contact with the infectious agent through placentas and birthing fluids from infected ewes and does. Older animals may be exposed to the prions this way as well. Colostrum and milk are also sources of prions. Other secretions such as urine, feces, saliva, and nasal secretions may contain infectious prions as well. Once ingested, the prions cross into the lymphoid system. The prions will incubate for a long time usually two to five years before entering the nervous system.
Genetics plays a part in Scrapie infections. Certain breeds are more susceptible to the disease due to genetic composition. Genetic testing is available for producers to help them select breeding stock with resistant genes.
Clinical signs most commonly associated with Scrapie are intense pruritis, ataxia, and wasting. Early in the disease, small ruminant producers may notice slight changes in behavior with sheep and goats infected with Scrapie. Initially, animals may have a staring or fixed gaze, may not respond to herding, and may be aggressive towards objects. As the disease progresses, other clinical signs noticed are progressive weight loss with normal appetite, incoordination, head tremors, and intense pruritis. In the terminal stages, sheep are recumbent and may have blindness, seizures, and an inability to swallow. Once initial clinical signs are notice, death usually occurs in one to six months.
The gold standard for postmortem (dead animals) diagnosing of Scrapie is the use of immunohistochemistry test on brain tissues as well as microscopic examination of brain tissue for characteristic TGE lesions. Live animal diagnosis is possible by testing lymphoid tissues from the third eyelid and rectal mucosa scrapings.
There is no treatment available for Scrapie, so prevention is key to controlling the disease. Following biosecurity protocols is a good starting point for preventing Scrapie. Part of the biosecurity plan is to maintain a closed flock and only buy replacement animals from certified Scrapie free flocks. Producers should limit visitors’ contact with their animals. Sanitation is important in lambing and kidding areas. Manure and bedding contaminated with birthing fluids and placentas should be disposed of properly. Genetically resistant animals should be used for breeding to produce genetically resistant offspring.
It should be noted that there is a novel or atypical form of Scrapie. This disease may also be referred to as Nor98 variant. This atypical version of Scrapie was initially found in Norway. It has been diagnosed in the United States as well. The disease is usually only found in a single old animal in the flock or herd. The brain lesions in atypical Scrapie are different from classical Scrapie. Currently, experts believe that natural transmission of atypical Scrapie is not likely.
The United States Department of Agriculture (USDA) has been battling Scrapie for decades. According to recent information from the USDA, the United States (US) is close to accomplishing eradication of the disease. In order for the United States to achieve Scrapie free status, no sheep or goats can test positive for classical scrapie for seven years and a certain level of testing needs to be done each year that represents the sheep and goat populations within the country. Small ruminant producers can assist the USDA eradication efforts by contacting the USDA when they have an adult sheep or goat exhibiting clinical signs of Scrapie or an adult animal dies or is euthanized. Producers should contact the Oklahoma State Veterinarian, Dr. Rod Hall at 405-522-6141 or the USDA Veterinary Services at 405-254-1797. This will aid the USDA in reaching sampling testing goals. There is no charge for the collection or testing of the samples for scrapie.
Scrapie is a disease that needs to be eliminated from the US. Once eliminated, the US will have additional export markets for sheep and goat products. Oklahoma State University Cooperative Extension Service has an informative fact sheet on Scrapie. Please visit the Local County Extension Office and asked for fact sheet VTMD-9135 or producers may view the fact sheet online at https://extension.okstate.edu/fact-sheets/scrapie.html. Also, the USDA National Scrapie Eradication Program website has valuable information as well at https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/sheep-and-goat-health/national-scrapie-eradication-program.
References Cassmann, E. D., & Greenlee, J. J. (2020). Pathogenesis, detection, and control of scrapie in sheep. American journal of veterinary research, 81(7), 600–614. https://doi.org/10.2460/ajvr.81.7.600
-

 Country Lifestyle7 years ago
Country Lifestyle7 years agoJuly 2017 Profile: J.W. Hart
-

 Outdoors6 years ago
Outdoors6 years agoGrazing Oklahoma: Honey Locust
-

 Country Lifestyle2 years ago
Country Lifestyle2 years agoThe Two Sides of Colten Jesse
-

 Attractions7 years ago
Attractions7 years ago48 Hours in Atoka Remembered
-

 Outdoors4 years ago
Outdoors4 years agoPecan Production Information: Online Resources for Growers
-

 Farm & Ranch6 years ago
Farm & Ranch6 years agoHackberry (Celtis spp.)
-

 Equine7 years ago
Equine7 years agoUmbilical Hernia
-

 Country Lifestyle1 year ago
Country Lifestyle1 year agoSay Yes!




